Electron beam induced deposition (EBID) uses the electron stimulated decomposition of volatile organometallics under low vacuum conditions to create nanostructures in a minimally-invasive, next-generation, maskless lithographic process. As a tool for nanofabrication, EBID offers an attractive and unique combination of capabilities including high spatial resolution and the flexibility to deposit free-standing three-dimensional structures on non-planar surfaces (Figure right). Despite the inherent advantages of EBID the deposits often contain unacceptably high level of organic contamination. These impurities negatively impact the properties (e.g. resistivity) and function (e.g. catalytic activity) of EBID nanostructures. Most of the contamination emanates from the electron stimulated decomposition of the ligand architecture that surrounds the central metal atom in the organometallic precursor. In the past several years we have used an array of surface analytical techniques (X-ray Photoelectron Spectroscopy, Mass Spectrometry, and Infrared Spectroscopy) to interrogate the sequence of electron stimulated bond breaking processes that occur within nanometer scale thick films of EBID precursors (e.g. MeCpPtMe3, Pt(PF3)4 and W(CO)6) under UHV conditions. Results from these studies have revealed that there a well-defined sequence of electron stimulated reactions which govern the decomposition of surface bound organometallic precursors which depend on the ligand architecture. This work has involved collaborations with Kees Hagen at TU Delft. Recently we have started to work with Professor Lisa McElwee-White at the University of Florida to use the “rules” of electron stimulated reactions we have uncovered in an effect to design organometallic precursors specifically for EBID.
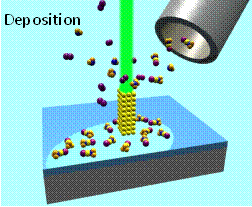
Electron beam induced deposition (EBID) of metal nanostructures from organometallic precursors.–courtesy of Ivo Utke (EMPA, Switzerland)